|
| ||||
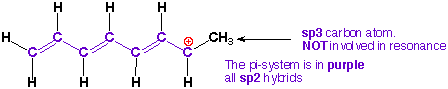
1) Identify the
pi-system in the molecule (adjacent sp and sp2 hybrid atoms).
2) Determine if the
pi-system is a cation, anion, radical, or neutral.

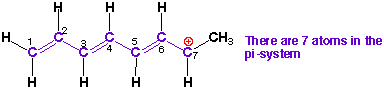
3) Count the total
number of atoms (p-orbitals) in the pi-system and number them.
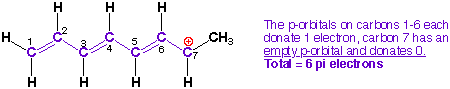
4) Count the total
number electrons in the pi-system (do not forget to include lone pairs and
radicals).
5) Draw a series of
p-orbitals in a line (if linear) or circle (if a ring) which have the same
number atoms as the pi-system (step 3). Draw the series once for every atom in
the pi-system. In other words, if
there are four p-orbitals, draw four series or four p-orbitals. The first atom
in the molecules pi-system will be represented by the first p-orbital in each
series. Each atom will correlate with one p-orbital.
6) Place all of the
pi-electrons (counted in step 4) into the orbitals in all possible combinations
using the following rules :
a. Place one electron in each orbital first. When all electrons have been placed, if
each orbital has only one electron (neutral or a radical), make each pi-system
identical.
b. If there is an
extra electron (anion), draw individual systems where the extra electron is in
a different orbital in each system.
c. If there are not enough
electrons and one orbital is empty (cation) draw systems where the empty
orbital is on a different atom in each system.
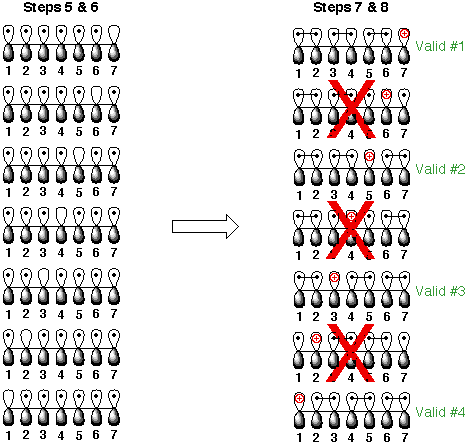
7) Draw bonds to
connect unpaired electrons in each pi system. These bonds represent the pi-bonds
in the resonance contributor. Remember: stable structures form the maximum
number of pi- bonds. Any pi system which has more than one unpaired electron
after the bonds are drawn is unstable and can be removed. All remaining structures are valid.
8) Place the charges
on the appropriate atoms (no bonds to other p-orbitals).
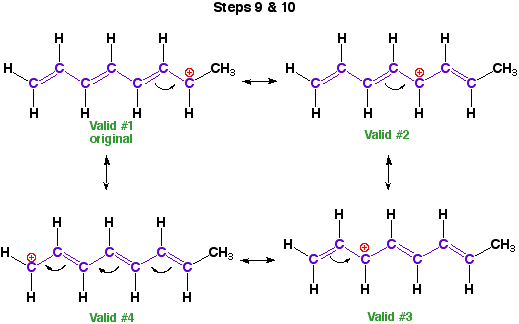
9) Superimpose the
valid pi-systems back on the original molecule.
10) Draw the arrows
to show the implied electron motion.
Place the resonance arrows between structures.