The Diels - Alder Reaction
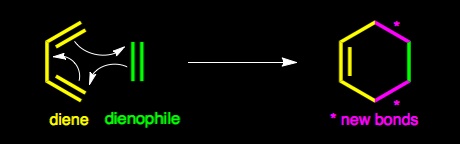
The Diels - Alder reaction is named after Otto Diels and Kurt Alder. In 1950 they were awarded the Nobel Prize for their work on this reaction. The Diels - Alder reaction is a cycloaddtion reaction between a two conjugated double bonds (a diene) and a carbon carbon double bond or triple bond (dienophile). The reaction is very important because two new carbon-carbon bonds and a cyclohexene ring are formed in a single step. The recation mechanism is pericyclic or concerted. In other words, the reaction is a single step process with no intermediates.
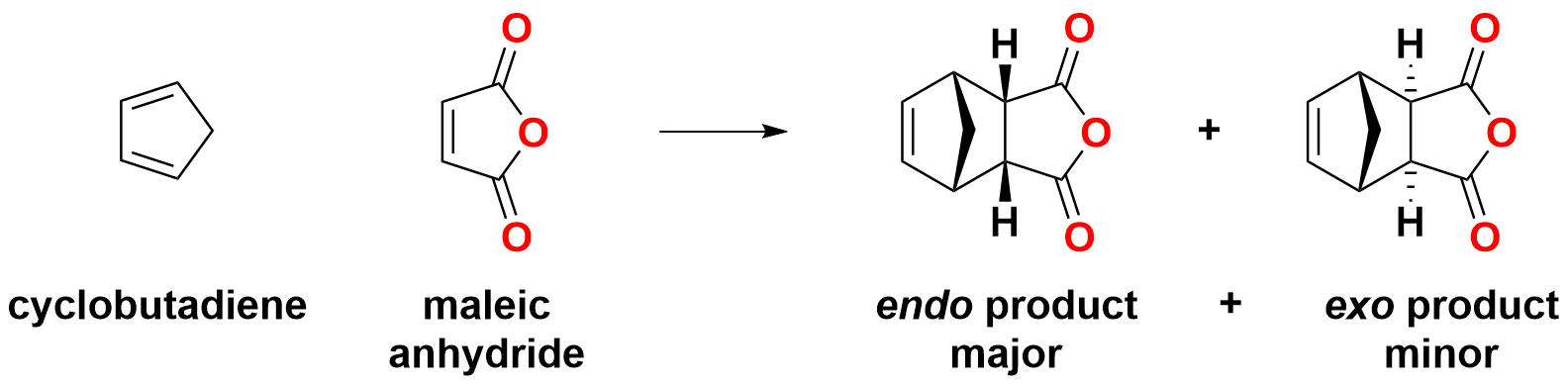
Below is an interactive model of a Diels-Aldert reaction between cyclopentadiene and maleic anyhydride. By selecting the atoms which form the new ring, you can better visualize how the reaction takes place. You may also rotate the molecule to get any perspective you like.
C = grey; H = cyan; O = red